News and Literature
News and Literature
Company News
- Company Mission: Solve unmet medical needs with innovative medicine
- R&D Focus: Novel medicines targeting the tumor microenvironment and ER Stress
- Clinical Development: ORIN1001, a First-in-Class IRE1α inhibitor, currently in Phase 2 trials in cancer and Phase 1b in IPF
- Leadership team: Drs. Qingping (Ted) Zeng, John Patterson and Stephanie Greene

Orinove Inc
News
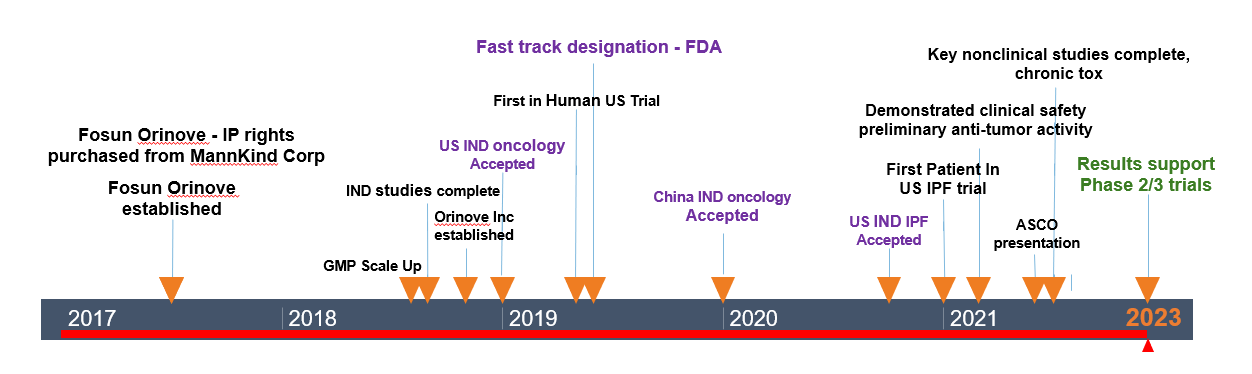
- November, 2018: Orinove Inc was established in Los Angeles
US subsidiary of Orinove (Orinove Inc) was established in Los Angeles on November 22, 2018. Both the Suzhou site and Los Angeles sites focus on small molecule anticancer drugs. Orinove Inc is mainly responsible for the core functions of the ORIN1001 clinical trials implementation in the US, CRO management and worldwide academic cooperation.
- January, 2019: FDA accepts IND to conduct the clinical trial with ORIN1001 in cancer patients
Orinove received FDA acceptance on January 28, 2019 to conduct the First in Human Phase 1 clinical trial of ORIN1001 for advanced solid tumors and relapsed refractory metastatic breast cancer in the US. ORIN1001 is a first-in-class small molecule with new enzyme-type targets, new mechanism of action and new chemical structure types.
- June, 2019: FDA grants Orinove Inc fast track approval for ORIN1001 in oncology
On June 16, 2019, the FDA granted Orinove Inc. fast track approval for ORIN1001 in oncology. The investigation of ORIN1001 for treatment of patients with relapsed refractory metastatic breast cancer is designated as a Fast Track development program.
- January, 2020: China FDA accepts IND to conduct clinical trials with ORIN1001 in cancer patients
- October, 2020: FDA accepts IND to conduct clinical trials with ORIN1001 in idiopathic pulmonary fibrosis (IPF) patient
- January, 2023: Phase 1 US clinical trial with ORIN1001 completed
Trial No.
ORIN1001-C1
ORIN1001-001
ORIN1001-002
ClinTrials No.
NCT05154201
NCT03950570
NCT04643769
Title
Treatment of patients with advanced solid tumors with oral agent, ORIN1001, and in combination with standard of care
ORIN1001 in patients with advanced solid tumors and relapsed refractory metastatic breast cancer
Evaluation of oral ORIN1001 in subjects with idiopathic pulmonary fibrosis (IPF) |
Oncology Clinical Sites: ORIN1001-001
California
UCLA Health Burbank Specialty Care
Burbank, California, United States, 91505
Principal Investigator: Sara Hurvitz, MD
UCLA Health Laguna Hills Cancer Care
Laguna Hills, California, United States, 92653
Principal Investigator: Sara Hurvitz, MD
University of California Irvine Medical Center (UCIMC) – Chao Family Comprehensive Cancer Center
Orange, California, United States, 92868
Principal Investigator: Parajuli Ritesh, MD
University of California Los Angeles (UCLA) – Jonsson Comprehensive Cancer Center (JCCC) – Oncology Center – Westwood
Westwood, California, United States, 90024
Principal Investigator: Sara Hurvitz, MD Colorado
University of Colorado Anschutz Medical Campus
Denver, Colorado, United States, 80045
Principal Investigator: Anthony Elias, MD
Highlands Ranch, Colorado, United States, 80129 Principal Investigator: Anthony Elias, MD
University of Colorado Lone Tree Medical Center
Lone Tree, Colorado, United States, 80124
Principal Investigator: Anthony Elias, MD
Florida
Coral Gables, Florida, United States, 33133
Principal Investigator: Frances Valdes-Albini, MD
Missouri
Kansas City, Missouri, United States, 64111
Principal Investigator: Timothy Pluard, MD
New York
Buffalo, New York, United States, 14203
Principal Investigator: Shipra Gandhi, MD
Northwell Heath Cancer Institute
New Hyde Park, New York, United States, 11042
Principal Investigator: Joseph Herman, MD
Northwell Health
New Hyde Park, New York, United States, 11042
Principal Investigator: George Raptis, MD
NYU Langone Health
New York, New York, United States, 10016
Principal Investigator: Douglass Marks, MD
Ohio
Canton, Ohio, United States, 44718
Principal Investigator: Nashat Gabrail, MD
Pennsylvania
Canton, Ohio, United States, 44718
Principal Investigator: Nashat Gabrail, MD
Thomas Jefferson University Hospital
Philadelphia, Pennsylvania, United States, 19107
Principal Investigator: Maysa Abu-Khalaf, MD
Tennessee
Nashville, Tennessee, United States, 37203
Principal Investigator: Erica Hamilton, MD
Texas
Houston, Texas, United States, 77030
Principal Investigator: Mothaffar Rimawi, MD
China Oncology Clinical Sites – ClinTrials.gov
Principal Investigator: Lin Shen, MD
Changchun, China, 130000
Jilin Cancer Hospital
Hangzhou, China, 310003
The First Affiliated Hospital, Zhejiang University School of Medicine
Harbin, China, 150000
Harbin medical University Cancer Hospital
Heilongjiang, China, 150000
Harbin Medical University Cancer Hospital
Jiangse, China, 215006
The First Affiliated Hospital of Soochow University
Jilin, China, 130000
Harbin Medical University Cancer Hospital
Jinan, China, 250000
Shandong Provincial Cancer Hospital
Shandong, China, 250000
Shandong Provincial Cancer Hospital
Shanghai, China, 200433
Shanghai Pulmonary Hospital
Suzhou, China, 215006
The First Affiliated Hospital of Soochow University
TianJin, China, 300060
Tianjin Medical University Cancer Institute and Hospital
Zhejiang, China, 310003
The First Affiliated Hospital, Zhejiang University School of Medicine
IPF Clinical Sites - Clintrials.gov
Florida
St. Francis Sleep, Allergy & Lung Institute
Clearwater, Florida, United States, 33765
Principal Investigator: Fran Averill, MD
Mayo Clinic Hospital
Jacksonville, Florida, United States 32224
Principal Investigator: Tarik Haddad, MD
Avanza Medical Research
Pensacola, Florida, United States, 32503
Principal Investigator: Luis Murillo, MD
Coastal Pulmonary and Critical Care
Saint Petersburg, Florida, United States, 33704
Principal Investigator: Warren Abel, MD
Illinois
Loyola University Medical Center
Maywood, Illinois, United States, 60153
Principal Investigator: Daniel Dilling, MD
Iowa
University of Iowa Hospital
Iowa City, Iowa, United States 52242
Principal Investigator: Nabeel Hamzeh, MD
Massachusetts
Infinity Medical Research
North Dartmouth, Massachusetts, United States, 02747
Principal Investigator: Curtis Mello, MD
Missouri
Hannibal Clinic
Hannibal, Missouri, United States, 63401
Principal Investigator: Humam Farah, MD
New Hampshire
Dartmouth Hitchcock Medical Center
Lebanon, New Hampshire, United States, 03766
Principal Investigator: Richard Enelow, MD
Ohio
University of Cincinnati
Cincinnati, Ohio, United States, 45220
Principal Investigator: Nishant Gupta, MD
North Carolina
Duke University Hospital
Durham, North Carolina, United States, 27710
Principal Investigator: Lake Morrison, MD
Our Technology
ORIN1001
ORIN1001 is a first-in-class small molecule targeting a novel enzyme with a unique mode of inhibition that selectively blocks the Inositol Requiring Enzyme 1α (IRE1) RNAse and blocks X-Box Binding Protein 1 (XBP1) activation in the endoplasmic reticulum.
Select Literature with ORIN1001:
Modulation of the proteostasis network promotes tumor resistance to oncogenic KRAS inhibitors. Science 2023, 381 (6662)
Targeting of BCR-ABL1 and IRE1α induces synthetic lethality in Philadelphia-positive acute lymphoblastic leukemia. Carcinogenesis 2020 9 11
The IRE1 and PERK arms of the unfolded protein response promote survival of rhabdomyosarcoma cells. Cancer Lett 2020 490,76-88
Local intracerebral inhibition of IRE1 by MKC8866 sensitizes glioblastoma to irradiation/chemotherapy in vivo Cancer Letters 2020, 494, 73-83
Pharmacological targeting of the unfolded protein response for disease intervention. Nature. 2019 15, 764-775
Inhibition of IRE1α RNase activity reduces NLRP3 inflammasome assembly and processing of pro-IL1β Cell Death & Disease 2019, 10(9), 622
IRE1α-XBP1s pathway promotes prostate cancer by activating c-MYC signaling Nat. Comm. 2019, 10(1), 323
Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy Nat. Comm. 2018, 9(1), 1-14
Pharmacological targeting of MYC-regulated IRE1/XBP1 pathway suppresses MYC-driven breast cancer” JCI 2018, 128(4), 1283-1299
Structure and mechanism of action of the hydroxy aryl aldehyde class of IRE1 endoribonuclease inhibitors Nat. Comm. 2014, 5, 4202
Disruption of microRNA Biogenesis Confers Resistance to ER Stress-Induced Cell Death Upstream of the Mitochondrion PLoS ONE 2013, 8(8), e73870
Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma Blood, 2012, 119(24), 5772-5781
Autophagy and the unfolded protein response promote profibrotic effects of TGF-b1 in human lung fibroblasts, Ghavami et al., Am J Physiol Lung Cell Mol Physio 2018
Localized hypoxia links ER stress to lung fibrosis through induction of C/EBP homologous protein, JCI Insight 2018; 3(16)
IRE1α–XBP1 signaling in leukocytes controls prostaglandin biosynthesis and pain. Science 2019, 365 6499.
