Technology
Our Technology
ORIN1001 is a novel and selective IRE1α RNAse inhibitor
ORIN1001 is an oral product and is currently in oncology clinical trials for the treatment of advanced solid tumors and metastatic breast cancer.
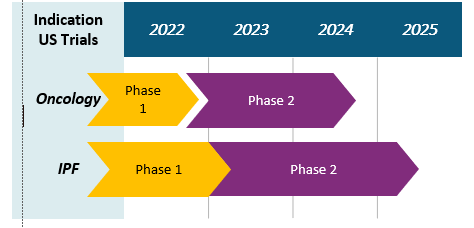
Phase 1 dose escalation is complete as a monotherapy and in combination with chemotherapy. A Phase 2 trial is poised to start.
Phase 1 oncology trials were conducted in the US and China.
A Phase 1b trial was conducted in the US in IPF patients.
IRE1 is thought to promote cell survival during conditions of cellular stress (for example, starvation, hypoxia, cancer and chemotherapy). In most healthy cells, the IRE1 pathway is not active until these stress conditions occur.

ORIN1001 is a first-in-class drug that provides an innovative approach to kill cancer cells by decreasing survival of cancer cells. ORIN1001 also decreases fibrosis in Idiopathic Pulmonary Fibrosis.
Our data indicate that ORIN1001 may be effective alone or in combination with standard of care, thereby facilitating the action of these other therapies, allowing use in expanded patient populations.
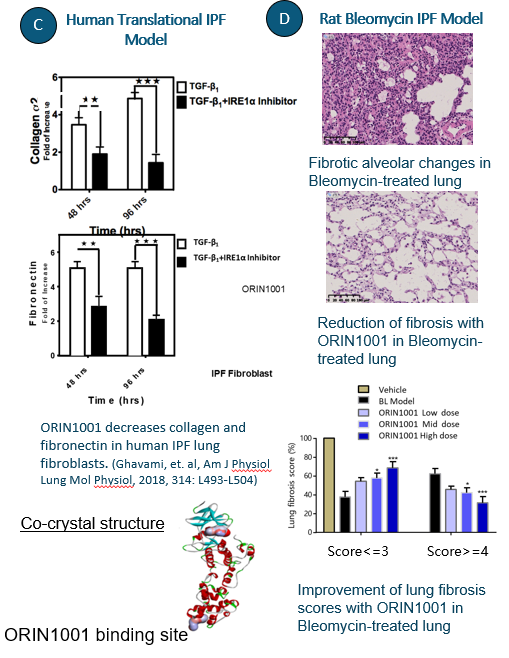
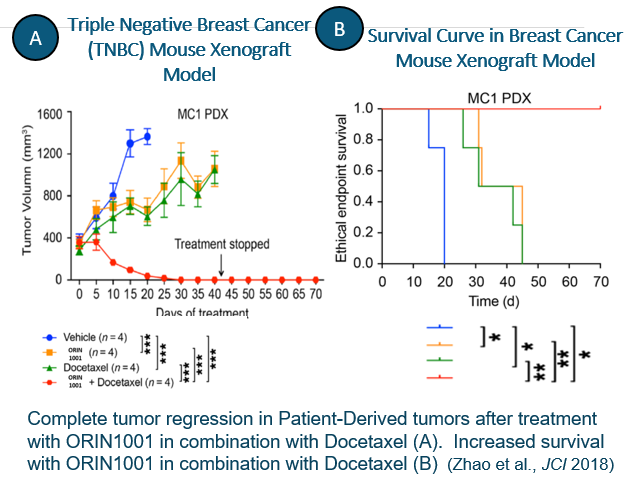
In preclinical models and in patient-derived xenografts, ORIN1001 has been shown to inhibit IRE1 activity and anti-tumor growth across multiple tumor types including breast, prostate, pancreatic, lung, liver, colon, esophageal and other solid tumors. ORIN1001 is an anti-fibrotic agent that shows marked efficacy in preclinical models of IPF.
- FDA Fast Track Designation granted.
- ORIN1001 demonstrates synergistic pre-clinical activity in combination with standard of care in multiple xenograft models of breast cancer and solid tumors, including prostate, liver, pancreatic, lung, ovarian, colon, esophageal and GBM.
- ORIN1001 demonstrates preclinical activity as a single agent in ex-vivo human IPF tissue studies and in traditional IPF preclinical in-vivo models.
- ORIN1001 is expected to enter Phase 2 clinical trials in oncology by YE2022 and in IPF by Q1 2023 with market exclusivity until 2037.
- ORIN1001 was initially developed as part of a joint venture with Fosun Pharma. Orinove is seeking global strategic partners for ORIN1001 in cancer and/or IPF.
World Leader in IRE1 target inhibition
Orinove Inc, a Thousand Oaks, California-based private company, is the world leader in IRE1 target inhibition for treatment of cancer and IPF.
- Highly potent and selective IRE1 inhibitor, ORIN1001, which blocks tumor survival pathway through multiple mechanisms, and reduces inflammation and fibrosis.
ORIN1001
The First Clinical Molecule to Target a Novel Pathway in the Treatment of Cancer and Idiopathic Pulmonary Fibrosis
Opportunity Highlights
- Orinove Inc. is the foremost expert in the IRE1 inhibition and has discovered and developed the only IRE1 inhibitor in clinical trials.
- The IRE1 pathway is a highly validated target and until now, has remained undruggable due to a lack of understanding of the pharmacodynamics and its relationship to the binding site and therapeutic indication.
- ORIN1001 demonstrates early clinical activity as a single agent and in combination with standard of care in advanced solid tumors. Best response was observed in one subject treated over 41 months. Multiple responders with late stage solid tumors.
- ORIN1001 demonstrates acceptable safety profile with monitorable, reversible side effects in >80 patients.
Orinove Advantage in Oncology:
- Clinically stable disease and partial response across multiple solid tumor types, including breast cancer
- Safety: Reversible, clinically monitorable side effects
- Oral tablet; No infusion
- Orphan Drug Exclusivity for Triple Negative Breast Cancer
ORIN1001 Advantage in IPF:
- Unmet medical need with no effective approved drug
- ORIN1001: No side effects or safety issues identified clinically
- Demonstrated Translational proof of concept
- Orphan Drug Exclusivity
Development Timeline and Intellectual Property:
- IP portfolio including composition of matter patents (2031) and combination treatment with international exclusivity through 2037
- Marketing Applications planned for US, China, EU and Japan
- Orinove is seeking strategic partners for the ongoing development of ORIN1001
ORIN1001 Clinical Trial Status :
- ORIN1001 Clinical Trial Status
- Phase 1: Oncology trial in China (14 clinical sites) – Completion Q1’23
- Phase 1b: IPF trial in the US (11 clinical sites) – In progress
- Phase 2: Oncology trial in the US (30 clinical sites) – Proposed start Q4’24
Orinove Management Team:
Dr Ted Zeng, CEO
Dr John Patterson, VP Translational Science
Dr Stephanie Greene, VP Clinpharm & Toxicology
